
In the pharmaceutical industry, maintaining the integrity of temperature-sensitive products is paramount. The cold chain refers to the series of processes and equipment used to ensure that pharmaceutical products are stored and transported at the correct temperature to maintain their efficacy and safety. This is crucial for a variety of medications, vacunas, and other healthcare products, as any deviations in temperature can compromise the quality and effectiveness of these products.
Managing the pharmaceutical cold chain involves a range of stakeholders including manufacturers, distributors, logistics providers, and healthcare facilities. Each of these parties plays a crucial role in maintaining the integrity of the cold chain and ensuring that pharmaceutical products reach patients in optimal condition.
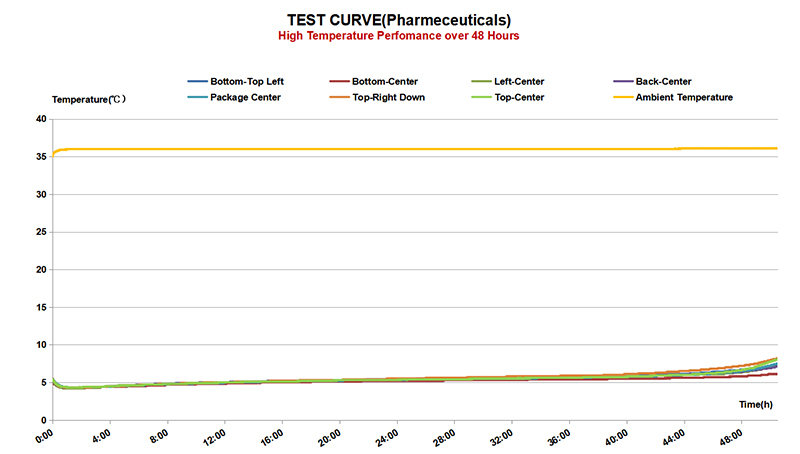
One of the key challenges in pharmaceutical cold chain management is the need for strict temperature control throughout the entire supply chain. From the moment a product is manufactured to the time it reaches the end-user, it must be kept within a specified temperature range to prevent degradation. This requires the use of specialized equipment such as refrigerated storage units, insulated packaging, and temperature monitoring devices to track and record temperature variations.
Another important aspect of pharmaceutical cold chain management is ensuring compliance with regulatory requirements. Regulatory bodies, such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe, have strict guidelines for the storage and transportation of pharmaceutical products. Failure to comply with these regulations can lead to the rejection of products or even legal consequences for the responsible parties.
En los últimos años, advancements in technology have led to improvements in pharmaceutical cold chain management. Por ejemplo, the use of temperature-sensitive labels and data loggers allows for real-time monitoring of products, giving stakeholders greater visibility into the conditions in which their products are being stored and transported. Además, the development of new packaging materials and insulation technologies has helped to better protect pharmaceutical products from temperature fluctuations during transit.
The importance of pharmaceutical cold chain management has been further highlighted by the global COVID-19 pandemic. With the urgent need for the distribution of vaccines to combat the virus, maintaining the integrity of the cold chain has been a critical factor in ensuring the effectiveness of these life-saving products. The rapid distribution of vaccines to millions of people around the world would not have been possible without the careful management of the cold chain.
Pharmaceutical cold chain management is essential for safeguarding the integrity of temperature-sensitive products throughout the supply chain. It requires collaboration and compliance from all parties involved, as well as the use of advanced technologies to monitor and maintain the correct temperature conditions. As the demand for pharmaceutical products continues to grow, the importance of effective cold chain management will only become more critical in ensuring the safety and efficacy of these products for patients worldwide.